U.S. researchers have found that slightly imperfect graphene can move protons (and only protons) from one side of the graphene membrane to the other in mere seconds thereby overcoming a major hurdle to this substance being used to build hydrogen fuel cells.
Researchers from Northwestern University discovered that if graphene naturally has a few tiny holes in it, what you have is a proton-selective membrane that could lead to a new and simpler mechanism for designing improved fuel cells. This research solves a major challenge in fuel cell technology to efficiently separate protons from hydrogen.
"Imagine an electric car that charges in the same time it takes to fill a car with gas," said chemist Franz Geiger, a professor of chemistry in the Weinberg College of Arts and Sciences who led the research, according to Phys.org.
"And better yet-imagine an electric car that uses hydrogen as fuel, not fossil fuels or ethanol, and not electricity from the power grid, to charge a battery. Our surprising discovery provides an electrochemical mechanism that could make these things possible one day."
Geiger said they've found if you just dial the graphene back a little on perfection, "you will get the membrane you want".
He noted that instead of working to make really pristine graphene, you need "less perfect graphene ... if you want to get protons through".
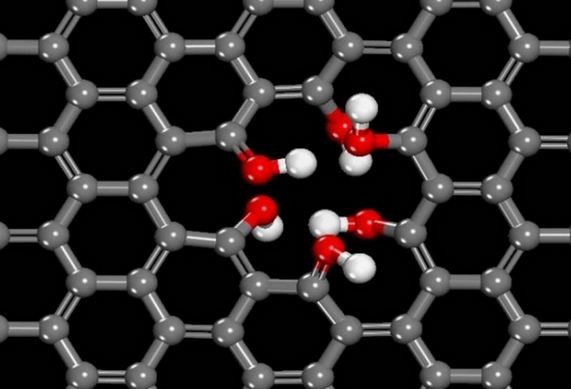
Graphene is a form of elemental carbon consisting of a single flat sheet of carbon atoms arranged in a repeating hexagonal, or honeycomb, lattice.
Researchers discovered that naturally occurring defects in the graphene, or one in which a carbon atom is missing, triggers a chemical reaction where protons from water on one side of the membrane are transferred to the other side in a few seconds.
They also found removing a few carbon atoms results in others being highly reactive, which starts the proton shuttling process. Only protons pass through the tiny holes, making the membrane very selective.
"Our results will not make a fuel cell tomorrow, but it provides a mechanism for engineers to design a proton separation membrane that is far less complicated than what people had thought before," Geiger said. "All you need is slightly imperfect single-layer graphene."
Geiger's research team included partners from Northwestern, Oak Ridge National Laboratory, the University of Virginia, Pennsylvania State University, the University of Minnesota and the University of Puerto Rico.