Researchers might have discovered what could be a groundbreaking treatment for children suffering from cystic fibrosis.
The new treatment reported by the Queensland Children's Medical Research Institute (QCMRI) in Australia combines two existing drugs that together ease lung infections in cystic fibrosis patients.
The Cystic Fibrosis Trust said the new therapy could "improve the lives of many".
QCMRI made the announcement after releasing results from the world's first clinical trials of the new treatment combining two existing drugs: ivacaftor and lumacaftor.
Ivacaftor (brand name Kalydeco) is a drug approved for patients with a certain mutation of cystic fibrosis that accounts for some five percent of cystic fibrosis cases. Lumacaftor (USAN, codenamed VX-809) is an experimental drug for the treatment of cystic fibrosis.
Both drugs were developed by Vertex Pharmaceuticals, a firm based in Boston, Massachusetts.
Neither drug, however, showed significant clinical outcomes when used alone. QCMRI interim director Claire Wainwright said the combination treatment resulted in up to 39 percent fewer lung infections, according to the Brisbane Times.
"Cystic fibrosis is the most common life-threatening genetic condition affecting children in Australia, so these results are incredibly exciting," she said.
"As well as the 30-39 percent reduction in lung infections, our trial participants reported a decrease in events requiring hospitalization or use of IV antibiotics.
"Results like this mean our novel treatment is a potential game changer for the way we treat CF and other genetic disorders in the future."
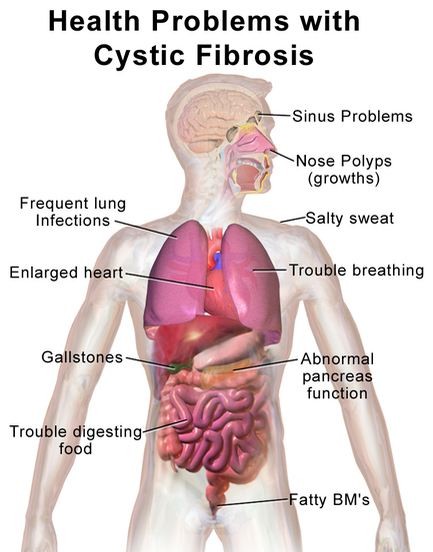
Cystic fibrosis sufferers have abnormally thick mucus linings in the lungs that clog airways and result in repeated infections and blockages.
Cystic fibrosis (CF), also known as mucoviscidosis, is a genetic disorder that affects mostly the lungs but also the pancreas, liver, kidneys and intestine. Long-term issues include difficulty breathing and coughing up sputum as a result of frequent lung infections.
The disease also affects the respiratory, digestive and reproductive systems and usually leads to a shortened life expectancy of some 37 years on average.
"Research into novel treatments like the one we trialed is essential to improve the quality of life for CF patients," said Prof. Wainwright.
She said there is still no cure for CF but with treatments like this proving successful, their research could have positive outcomes for CF patients not just here in Australia but internationally for years to come.
More than 1,100 children under the age of 12 took part in the six-month study involving 187 hospitals in Australia, Europe and the United States and consisting of two randomized, double-blind, phase 3 clinical trials.
The results of the trial reported in the New England Journal of Medicine showed this combination of drugs could bypass the genetic errors that cause cystic fibrosis and may increase life expectancy.
Founded in 1964, the Cystic Fibrosis Trust is the United Kingdom's only national charity dedicated to all aspects of CF. It funds research to treat and cure CF and aims to ensure appropriate clinical care and support for people with cystic fibrosis.