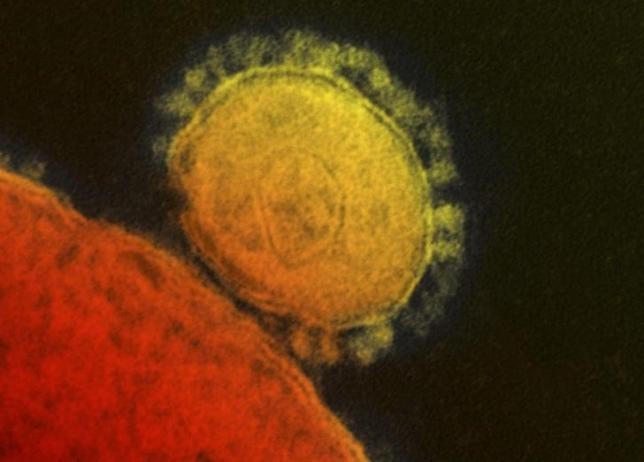
Three years after the Middle East respiratory syndrome (MERS) surfaced in Saudi Arabia it has infected 1,227 or more people and killed one-third of them; and recently resulted in 186 infections in South Korea. No prescription drugs exist for the virus' treatment. The first medications and vaccines will be based on both public policy and scientific research, and their development will be challenging due to the disease not being an epidemic.
For example, a new study published on July 27, Monday in the Proceedings of the National Academy of Sciences reveals that antibodies (virus-neutralizing proteins) from a MERS survivor's blood can effectively treat the viral disease in mice. That is the good news.
However, there is an asterisk. Clinical trials lasting up to a decade would be required to prove that the antibody is safe and effective in people.
MERS results from a coronavirus like the one that triggers Severe Acute Respiratory Syndrome (SARS). One decade ago China's government killed 10,000 civets (small nocturnal animals) as scientists believed they transmitted the disease to humans, according to Wired.
Scientists' theory is that camels spread MERS to humans. However, because Middle Eastern camels have high economic and cultural values, only an effective drug or vaccine could end the microbes' spread.
Antibodies are the most promising candidate. Identifying them is easy, and scientists are now focusing on extracting them from MERS patients.
Antonio Lanzavecchia of ETH Zürich led the latest study. He shared that after extracting blood from a patient, his team produced antibodies in just four months.
However, long clinical trials are another story. Thus, other researchers are looking at alternative MERS treatments, such as existing FDA-approved medications and virus vaccines.
MERS has not reached an epidemic level. This situation limits research funding for a cure, yet the recent outbreak highlights the need to develop one.
South Korea declared on July 28, Tuesday that it was in effect safe from MERS. The official announcement was made over two months after the first case of the disease was reported, and it then spread to 36 people in hospital environments, according to NBC News.