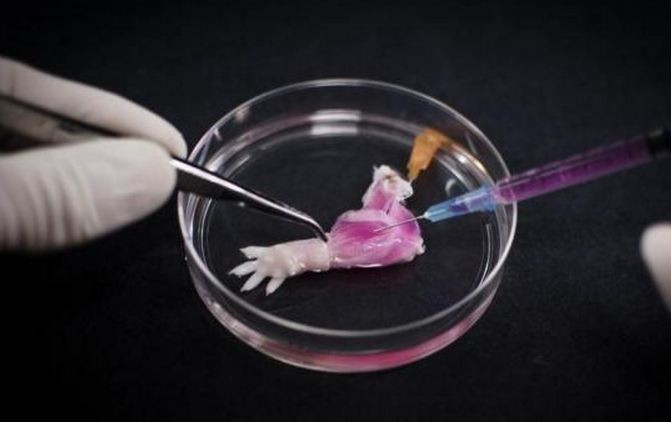
The success of a research team from Massachusetts General Hospital in being the first to grow a rat forelimb in a laboratory is seen as a huge step towards the development of "bioartificial replacement limbs" suitable for transplantation.
Researchers said this first step used an experimental approach previously used to build bioartificial organs to engineer rat forelimbs with functioning vascular and muscle tissue. They also provided evidence the same approach could be applied to the limbs of primates like human beings.
In this technique, new muscles and veins from the limb of a dead rat were grown before the limb was successfully implanted into a living rat. Researchers stripped cells from the dead rat's vascular and nerve matrix and regrew muscles, veins and arteries. This technique has been used before to successfully regenerate organs.
Muscle fibers of the regrown rat forelimb were electrically stimulated, causing them to contract with 80 percent of the strength a newborn animal would have. Researchers also said blood flowed through the limb successfully when it was transplanted to a live animal.
"The composite nature of our limbs makes building a functional biological replacement particularly challenging," said Harald Ott, MD, of the MGH Department of Surgery and the Center for Regenerative Medicine and senior author of the paper published in the journal Biomaterials.
"Limbs contain muscles, bone, cartilage, blood vessels, tendons, ligaments and nerves -- each of which has to be rebuilt and requires a specific supporting structure called the matrix. We have shown that we can maintain the matrix of all of these tissues in their natural relationships to each other, that we can culture the entire construct over prolonged periods of time, and that we can repopulate the vascular system and musculature".
Over 1.5 million individuals in the U.S. have lost a limb, said the study. Prosthetic technology, while quite useful, has many limitations in both function and appearance.
The current study uses technology discovered by Dr. Ott as a research fellow at the University of Minnesota. In this decellularization technique, living cells are stripped from a donor organ with a detergent solution. The remaining matrix is then repopulated with progenitor cells appropriate to the specific organ.
Dr. Ott's team and others at MGH and elsewhere have used the decellularization technique to regenerate kidneys, livers, hearts and lungs from animal models.
But this is the first reported use to engineer the more complex tissues of a bioartificial limb.
Dr. Ott said while regrowing nerves within a limb graft and reintegrating them into a recipient's nervous system is one of the next challenges that needs to be faced, the experience of patients that have received hand transplants is promising.
"In clinical limb transplantation, nerves do grow back into the graft, enabling both motion and sensation, and we have learned that this process is largely guided by the nerve matrix within the graft. We hope in future work to show that the same will apply to bioartificial grafts. Additional next steps will be replicating our success in muscle regeneration with human cells and expanding that to other tissue types, such as bone, cartilage and connective tissue."
Ott is an assistant professor of Surgery at Harvard Medical School. Bernhard Jank, MD, of the MGH Center for Regenerative Medicine is lead author of the Biomaterials paper.
Additional co-authors are Linjie Xiong, MD, Philipp Moser, MD, Jacques Guyette , PhD, Xi Ren, PhD, and Leopoldo Fernandez, MD, MGH Center for Regenerative Medicine and Curtis Cetrulo, MD, David Leonard, MBChB, and Shawn Fagan, MD, MGH Department of Surgery. The study was supported by a New Innovator Award from the National Institutes of Health.